The eight inos of this group are precipitated as sulfides or hydroxides by a combination of hydrogen sulfide or thioacetamide with an ammonia-ammonium chloride buffer. None of these ions form chlorides, sulfides, or hydroxides sufficiently insoluble to precipitate from a solution that is 0.1 to 0.3 M in hydrochloric acid.
Al+++, Cr+++, Fe+++, Fe++, Mn++, Co++, Ni++, Zn++
Outline
Original Sample:
Precipitate of the Group:
Dilute the sample to volume is about 2 mL.
Add 6 drops 6M NH4Cl ; 15M NH3 . Add 4 drops TA solution. And boil it for 5 minutes.
Precipitation formed after added TA solution
Centrifuge the solution, and use NH4NO4 solution to wash the precipitation. The solution is for the test of Cation Group 4. Separate the solution and precipitation.
Add 10 drops 16M HNO3 to dissolve the precipitation. Centrifuge the solution and discard precipitation.
Precipitation is floating on hot nitric acid solution
Solution 1
Use gentle fire to
vapor it with 15 drops 15M nitric acid (do not heat it to dry). Repeat the step three
times.
Drop out about 1~2 drops of Solution 1 to do Mn Test 1
Mn Test 1
Dilute 1 drop of
sample with several drops of water. Add some NaBiO3.
The Right One is Mn Test 1
Boil Solution 1, and add
some KClO3 (to raise the oxidation state of manganese) and some nitric acid until black precipitation forms.
Black precipitation at the crucible with some nitric acid
Wash with some water, centrifuge the solution and separate solution from precipitation.
And label the solution as solution 2, precipitation as precipitation 2.
Yellow solution show the sample contain Fe+++
Mn Test 2
Add 6 drops HNO3 16M and 1 drop H2O2 into the precipitation 2. And then add NaBiO3 some powder.
The Left One is the Mn test 2
Add 10 drops 6M
NaOH(aq) into the Solution 2. And 1 drop 3% H2O2. Put it into hot bath for 5 minutes. Centrifuge
and wash precipitation with 10 drops water and 1~2 drops 6M NaOH solution. Collect all liquids and centrifuge them together. Separate solution from precipitation and label solution as solution 3, precipitation as precipitation 3.
Hydroxide precipitation formed
Precipitation 3
Add 6 drops HCl 12M to dissolve the precipitation 3
and dilute it with 10 drops water. Divide the solution between two test tubes. One for Ni test and one for Fe, Co test.
Fe Test
Add some NH4SCN in to one of the test tube to react with the solution. A blood red color show the existence of of Fe.
Iron ion formed complex with thiocyanate ion
Add some NaF powder in to the solution to quench out the color of the complex and thiocynate ion.
Fluoride ion would react with iron ion and
form insoluble salt iron fluoride precipitation
Co Test
And then add some drops ammonium thiocyanate solution of ethanol-ether into the test tube. Cobalt ion would form a greenish color complex in the organic layer(ethanol-ether layer).
A greenish color shows the existence of Co ion
Ni test
Take another solution sample, and add some ammonia solution to alkalize the solution.
Add drops DMG(dimethylglyoxime) solution. The solution turn red shows the existence of nickel ion.
Looks like grape fruit juice color shows the existence of Ni ion
Solution 3
Divide solution 3 into three parts, one for Cr test, one for Al test and one for Zn test.
Cr test
Add some acetic acid and drops lead acetate into one part of the solution.Yellow precipitation form. It would be PbCrO4 .
Yellow precipitation would be PbCrO4
Ensure the yellow precipitation is lead chromate, centrifuge the solution to separate the precipitation from the solution.Add 1 drop 6M HNO3 and 1 drop 3% H2O2 into precipitation.
A blue color would form immediately and vanish in a second. It is supposed to be CrO5 .
It is one kind of a very unstable state of chromium oxide.
Blue color forms and vanishes just in seconds
Acidify the other two parts of solution with 6M HCl add 5 drops 6M NH4Cl and
NH3 to basic. Centrifuge it and separate the solution from the precipitation.
Aluminum hydroxide form a white precipitation
Al Test
Dissolve the precipitation in 6M HCl solution, add 2 drops aluminon agent and NH4OAc .
A cranberry color formed, it show the existence of Al ion
Zn Test
Add 2~3 drops BaCl2 in to the solution, centrifuge it. Discard precipitation. Add some drops of
TA solution and centrifuge it to take out the precipitation. Dissolve in HNO3 and add some drops NaOH. Test the solution by dithizone
paper.
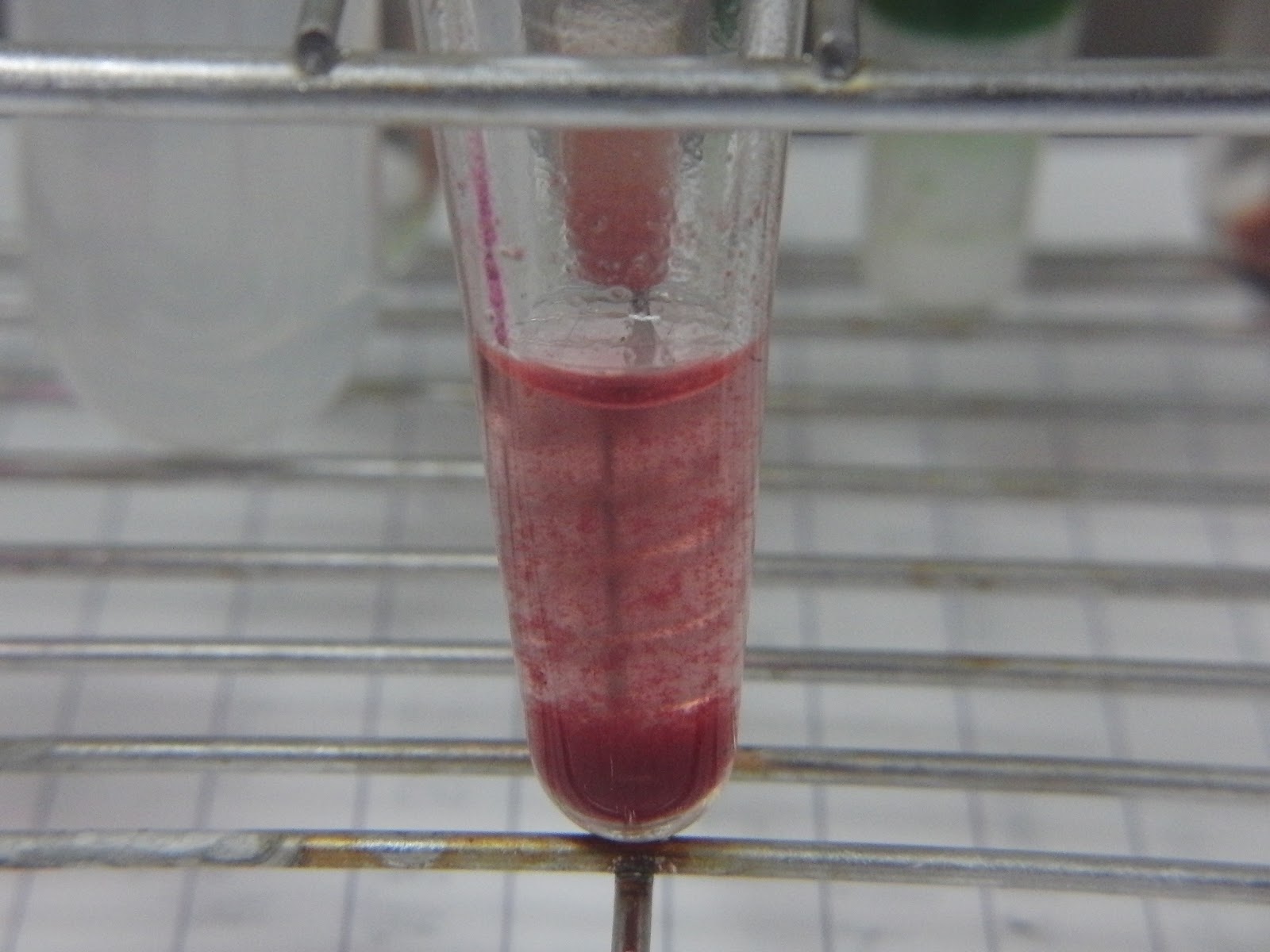
Dithizone would form complex with Zn ions,
the complex looks like cranberry red
References:
1. Qualitative Analysis and Electrolytic Solution
2. Introduction to Semimicro Qualitative Analysis (8 ed.)

















No comments:
Post a Comment