Cation
Group 2 – The Hydrogen Sulfide Group
Hg++, Cd++, Cu++, Pb++, Sn++, Sn(IV),
Bi+++, Sb(III), Sb(V), As(III), As(V)
The eight elements of this
group form sulfides that are precipitated by hydrogen sulfide or thioacetamide
from solution that are that are 0.1 to 0.3 M in hydrochloric acid. Of the
chloride only that of lead is sparingly soluble in dilute hydrochloric acid and
precipitates with Cation Group 1 to an appreciable extent. Silver and
mercury(I), had they not already been removed as chlorides, would precipitate
as sulfides with Group 2.
Thioacetamide is a white crystalline solid. It is soluble in water and serves as a
source of sulfide ions in the systemic analysis of Group 2.
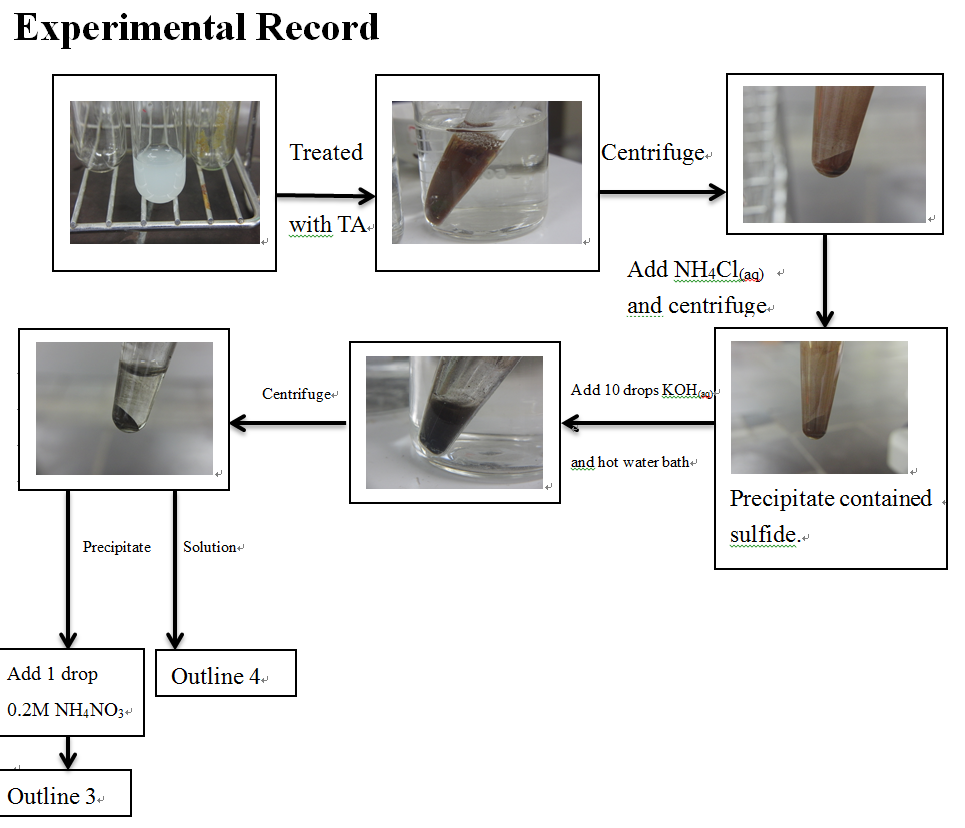
TA (Thioacetamide) solution in pH 0.5
Outline
1
Testing sample
Warm the solution containing TA at pH 0.5 in a bath of
vigorously boiling water for 5 minutes.
After added TA solution into testing sample, warmed in water bath
Centrifuge and withdraw
solution to another test tube.
Check the acidity with
short range indicator paper. Add 2 drops TA and heat for several minutes in the
boiling water. Centrifuge if more precipitation appears. Continue heating with
TA as long as precipitation is obtained. Then dilute several drops of the clean
solution with twice its volume of water, add 1 drop TA, and heat again. If a
precipitation appears, treat the whole solution in the same way; divide it
between several test tube if necessary.
Combine precipitation if
several portions have been obtained. Wash the precipitated sulfides twice with
about 20 drops hot water containing 1 drop 6M NH4Cl. Add the first
washing to solution2-1 in a general analysis for all groups.
Solution 2-1
Add 10 drops 0.5M KOH and heat
briefly in the water bath. Centrifuge and transfer the solution to a centrifuge
tube. Repeat the extraction with a second portion of KOH solution. Combine
solutions.
Precipitation 2-2
Wash twice with hot water to
which either 1 drop 0.2M NH4NO3 or crystal of the solid
salt has been added.
Analysis according to Outline 2
Solution 2-2
Recentrifuge to remove the last
traces of precipitation. Transfer the clear, yellow solution to a test tube.
Analysis according to Outline 3
Outline 2
Treat precipitation with 5
drops water and an equal volume 6M nitric acid. Warm it in water bath until
reaction occurs. Centrifuge and wash precipitation with water. Add wash to
solution3-1.
Solution 3-1
Transfer to a casserole and add
2 drops 18M sulfuric acid. Evaporate over flame and directly under a
ventilating duct or in the hood until white fume of SO3 are
copiously evolved. Cool for a while. Use many drops of water to wash to
casserole to collect the solution. Transfer the solution quickly to a
centrifuge tube. Centrifuge it.
Precipitation 3-1
Add a few drops aqua regia and
warm. Transfer solution to a crucible. Evaporate over a microflame to a small
volume but not to dryness. Dilute with about 1 mL water and transfer to a
centrifuge tube. Centrifuge if not clear and discard residue. Add SnCl2
solution.
White precipitation turning
gray:
Hg + Hg2Cl2: Presence of Hg(II)
Solution 3-2
Add 15M NH3 until is
alkaline.
Centrifuge it.
Blue solution shows copper(II) ions
Precipitation 3-2
Warm it with a few drops NH4OAc
solution. Centrifuge and discard residue. To solution add 1 drop 6M HOAc and a
few drops K2CrO4
Yellow precipitation: Pb CrO4
presence of Pb.
Precipitation 3-3
Add sodium stannite solution.
Jet black precipitation(Bi0):
Presence Bi
Bismuth formed in the solution
Solution 3-3
Add some Na2S2O4.
Warm it for 1~2 minutes. Centrifuge quickly and remove solution. Repeat the
treatment again. Add some drops TA and warm it.
Yellow precipitation(CdS):
Presence of Cd
Outline 3
Add 1 drop TA and
some 2M HCl. Centrifuge the solution, Draw off the solution as possible.
Precipitation 4-1
Add 5~10 dros 12M
HCl and warm it in hot water bath. After reaction occurred, centrifuge the
solution.
Precipitation 4-2
Add 2 drops 15M NH3
and 1 drop 3% H2O2. Warm it and centrifuge. Discard
residue. To solution add several drops of magnesia mixture. Let it react 5
minutes. Centrifuge and discard solution. Wash once with water.
Precipitation 4-3
Add 1 drop 6M HOAc
and several drops AgNO3 solution.
Reddish brown precipitation:
Ag3AsO4
Presence of As
Solution 4-2
Evaporate the
solution to half the original volume. Dilute with about 1 mL water and divide
between two test tubes.
Sb Test
Add some oxalic
acid and a few drops TA.
Deep orange: Sb2S3 ; presence of
Sb.
Sn Test
Add some drops of
HCl and a peace of Al. Warm the solution in hot water bath for some minutes.
Centrifuge the
solutiton.
Precipitate 4-4
Black precipitation: Presence Sb0
Solution 4-4
Add 1~2 drops HgCl2
solution.
White precipitation (Hg2Cl2): Presence of Sn
Experimental Record























No comments:
Post a Comment